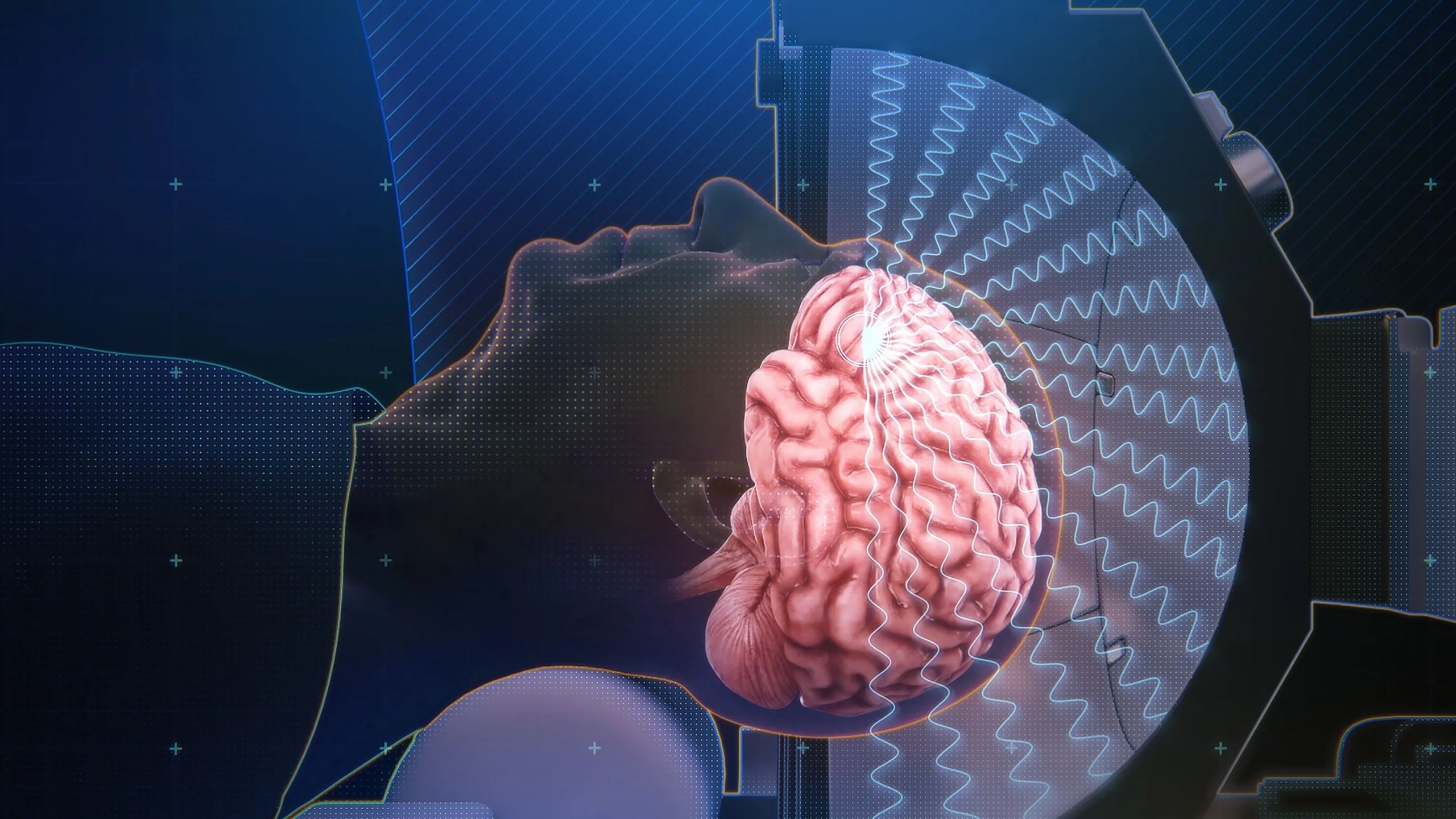
This study represented one of the first opportunities to directly assess the safety of transcranial focused ultrasound neuromodulation in human brain tissue using a clinically deployed system. Focused ultrasound was delivered using the BrainSonix BX Pulsar 1002, allowing investigators to evaluate safety under parameters representative of real-world neuromodulation protocols. By enrolling patients with medically refractory temporal lobe epilepsy who were already scheduled for surgical resection, the authors uniquely positioned themselves to examine post-sonication tissue histology rather than relying solely on imaging or clinical observation.
Resected tissue was analyzed for evidence of hemorrhage, necrosis, inflammation, or structural disruption, and the investigators reported no histopathological abnormalities attributable to ultrasound exposure. This finding provided rare and powerful confirmation that low-intensity focused ultrasound, when delivered within controlled parameter ranges, did not produce detectable tissue injury in humans. At the time of publication, this study stood among the strongest direct human safety validations of ultrasound neuromodulation, complementing prior animal and imaging-based work and reinforcing confidence in advancing ultrasound neuromodulation into sensitive clinical populations.